Does Tapping on the Top of Cans Work
Does tapping a can of fizzy drink actually cease it foaming over?

It is i of the distinct sounds of summer: the noise of people tapping the tops of their cans of fizzy drink before opening them. But does this widespread ritual really stop a tin can of beer or pop from gushing over?
When y'all open a can of fizzy drink, the refreshing "hiss" is the upshot of gas bubbles escaping from the liquid equally a event of a alter in the solubility of the carbon dioxide (CO2) in it. This change occurs due to the pressure level inside the tin decreasing from ~three bar (tin airtight) to ane bar at atmospheric pressure (tin can open). The solubility of CO2 in water reduces from ~4.5g in one litre of water at ~3 bar, to ~one.5g at atmospheric pressure, something that is described by Henry'south Police.
Before the can is opened, microscopic gas bubbling attach to the inside of it (nucleation). When the tin can is opened, these bubbling increment in size, due to the decrease in the solubility of CO2. When these bubbles reach a certain size they detach from the inside of the can and rise upwards to the top of the can due to buoyancy and displace liquid in their path (as shown in Figure 1).

And then what part could tapping the top of the tin can play in this process? Whether or non this technique really works is the subject area of some debate merely there is a theory explaining why information technology may work. As described before, the bubbles in an unopened tin can nucleate at the walls (Figure 2a) so tapping the can before opening could dislodge some of the bubbles, enabling them to float to the top of the liquid.
When a tin can is opened, the bubbles aggrandize (Figure 2b) with those deeper within the liquid travelling farther than those near the surface, displacing more of the drink and maybe resulting in greater amounts of ejected liquid. A "tapped" can will have fewer of these "deep" bubbles and so less liquid will be dislodged – and possibly sprayed out – than an "untapped" can (Effigy 2c).
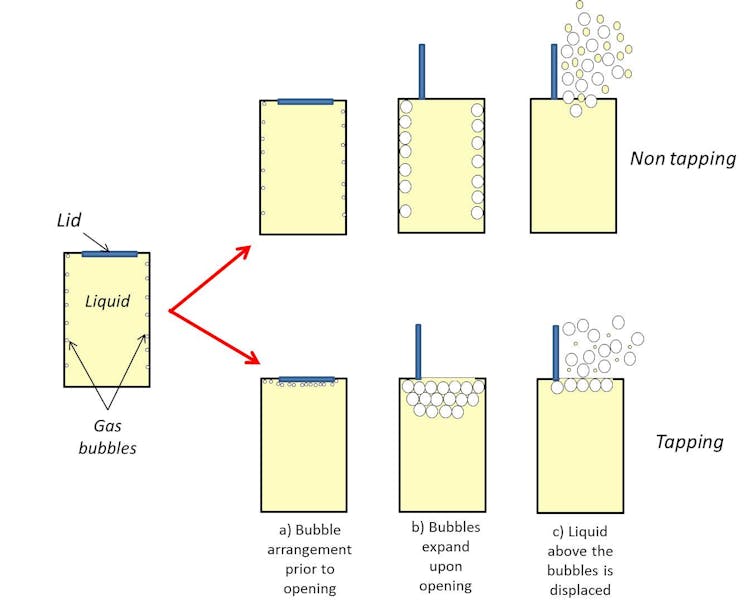
Bubbles also tin be dislodged from the side of the can with fierce shaking, of form – simply this method introduces more turbulence which increases the energy of the organization, resulting in more bubbles in the drink and more spraying when opened. Sharply borer the top of an open beer canteen with another has a similar issue, commonly resulting in a jumbo gush of beer foam. This is because pressure level waves acquired by the touch on create tiny "mushroom clouds" inside the bottle that eject huge quantities of liquid equally they escape.
Glass and gushing
The debate of tapping bated, the bodily material that the container is made from may besides reduce gushing. It has been shown that the corporeality of cream formed when pouring beer into glasses of different "wettabilities" – the extent to which h2o wets a fabric – tin bear on not only the amount of beer head formed just also the size of the bubbles on the inside of the glass. This information is relevant when such bubbles are thought to exist the crusade of gushing.
Another important gene when information technology comes to the level of gushing is the stabilisation of the bubbling caused past the presence of large molecules in the beverage. This is why some beers accept long-lived foam heads compared to the brusk-lived bubbles at the surface of, say, sparkling water. But such cream stabilising agents are a chat for some other day.
So this summertime why not attempt unlike ways of opening your fizzy potable – and see how much of it y'all terminate upwardly wearing.
Pembaca kami
Jumlah pembaca The Conversation sebanyak 18 juta pengguna setiap bulan, dan melalui Creative Commons republikasi menjangkau 42 juta pembaca.
Mau menulis?
Tulis artikel dan bergabung dengan komunitas akademisi dan peneliti yang terus tumbuh dengan lebih dari 141.300 dari 4.282 lembaga.
Daftar sekarang
Source: https://theconversation.com/does-tapping-a-can-of-fizzy-drink-really-stop-it-foaming-over-60659